Haemoglobin disorders are the most common genetic conditions found in humans, with 7% of the population estimated to be carriers and more than 330,000 infants being born with one of these disorders every year (RACGP 2023; ALEC 2025).
What are Haemoglobin Disorders?
Haemoglobin disorders, also known as haemoglobinopathies, are inherited conditions that affect haemoglobin (Hb) - a protein in the red blood cells responsible for transporting oxygen around the body (GOV.UK 2025).
These disorders are caused by a mutation in the genes responsible for coding haemoglobin (ALEC 2025).
There are over 1000 known gene mutations that can cause haemoglobin disorders, ranging from those that are clinically insignificant to others that have the potential to cause serious illness (GOV.UK 2025).
Haemoglobin disorders include:
- Those that cause insufficient haemoglobin production
- Thalassaemias
- Those that cause structurally abnormal haemoglobin production
- Sickle cell disease
- Hb C
- Hb D
- Hb O-Arab
- Hb Lepore
- Hb E.
(GOV.UK 2025)
Among the infants born with a haemoglobin disorder, most inherit sickle cell disease (83%), while about 17% inherit thalassaemias (ALEC 2025).
Risk Factors for Haemoglobin Disorders
The following groups of people are more likely to be carriers of haemoglobin disorders:
- People with a family history of anaemia or haemoglobin disorders
- People from certain ethnic backgrounds where carriers are more prevalent, including:
- Southern European
- African
- Middle Eastern
- Chinese
- Indian subcontinent
- Central and south-east Asian
- Pacific Islander
- New Zealand Maori
- South American
- Caribbean
- Certain Aboriginal and Torres Strait Islander communities in northern Western Australia and the Northern Territory
- People who have either:
- An average corpuscular volume (MCV) of under 80 fL
- Average corpuscular haemoglobin (MCH) of under 27 pg.
(RACGP 2023)
How are Haemoglobin Disorders Inherited?
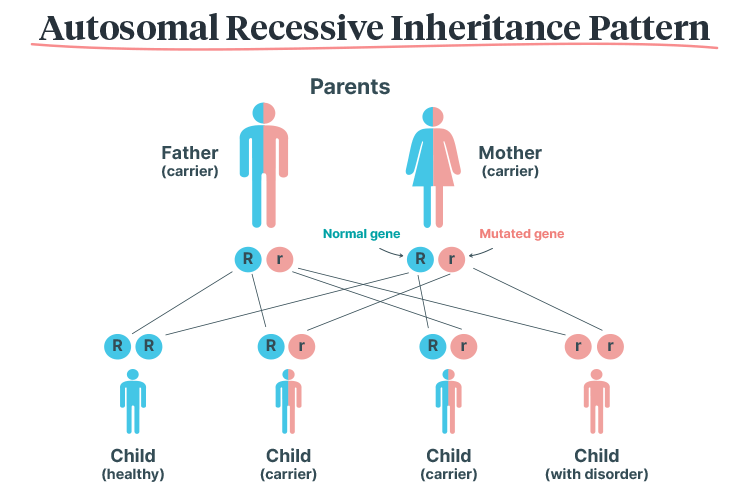
Haemoglobin disorders have an autosomal recessive inheritance pattern, meaning that they can only be passed on if both parents carry the mutated gene. The child must inherit two copies of the mutated gene (one from each parent) in order to inherit the disorder. Those who only inherit one copy become carriers (RACGP 2023).
Therefore, if both parents are carriers:
- There is a 25% chance that the child will be unaffected
- There is a 50% chance that the child will be a carrier
- There is a 25% chance that the child will have a haemoglobin disorder.
(Mayo Clinic 2024)
Diagnosis of Haemoglobin Disorders
Haemoglobin disorders can be diagnosed through blood tests and genetic testing (Better Health Channel 2019).
Screening for Haemoglobin Disorders
Couples planning a pregnancy can undergo genetic testing to determine whether they are carriers of a haemoglobin disorder (RACGP 2023).
The Royal Australian and New Zealand College of Obstetricians and Gynaecologists recommends that all patients undergo full blood examination (FBE) for MCV and MCH prior to trying for a pregnancy (or as early as possible in pregnancy). Depending on the results of these tests, as well as other factors such as ethnic background and family history, additional tests may be performed (ALEC 2025). These include:
- Ferritin blood test to exclude iron deficiency
- Haemoglobin electrophoresis test
- DNA analysis, if required.
(ALEC 2025)
Currently, newborn screening for haemoglobin disorders is not performed in Australia (RACGP 2023).
Types of Haemoglobin Disorders
Thalassaemias
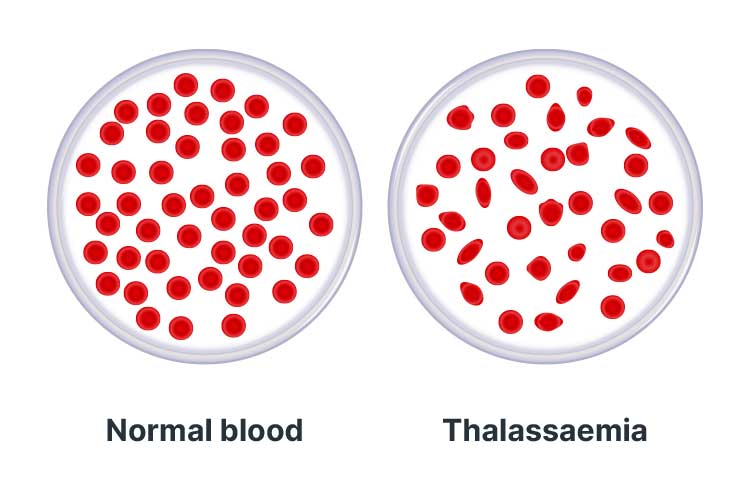
Thalassaemias are a group of haemoglobin disorders that cause insufficient production of haemoglobin, impairing its ability to transport oxygen around the body (GOV.UK 2025).
This means that red blood cells are only able to survive for a few weeks (as opposed to the usual duration of four months), and consequently, cause reduced levels of oxygen in every cell of the individual’s body (Better Health Channel 2019).
There are two types of thalassaemia:
- Alpha-thalassaemia (α-thalassaemia), which is caused by changes to both the HBA1 and HBA2 genes
- Beta-thalassaemia (β-thalassaemia), which is caused by changes to the HBB gene.
(Better Health Channel 2019)
Thalassaemias are classified as either:
- Thalassaemia minor, which is caused by one copy of the mutated gene(s) (i.e. the individual is a carrier), or
- Thalassaemia major, which is caused by two copies of the mutated gene(s) (i.e. the individual experiences symptoms as they have no functional copies of the affected gene).
(Better Health Channel 2019)
If left untreated, thalassaemia major can be fatal (Better Health Channel 2019).
Symptoms of Thalassaemia Major
- Severe anaemia
- Pale skin
- Sleeping difficulties
- Reduced appetite
- Slow weight gain/faltering growth/failure to thrive
- Enlargement of organs (e.g. spleen and liver).
(Better Health Channel 2019)
Treatment for Thalassaemia Major
The only known potential cure for thalassaemia major is a bone marrow transplant. However, this is associated with serious risks (Better Health Channel 2019).
Despite this, thalassaemia major can be managed through regular blood transfusions alongside iron chelation therapy to remove excess iron from the blood (Better Health Channel 2019).
Bart’s Hydrops Fetalis
Alpha-thalassaemia major, also known as Bart’s Hydrops Fetalis, occurs when no functional alpha-globin genes are inherited (i.e. all four copies of HBA1 and HBA2 are mutated) (GOV.UK 2025).
This is a serious condition that causes anaemia in the fetus and is generally incompatible with life outside of the uterus, leading to stillbirth or death soon after birth. In rare cases, some infants survive through intrauterine transfusions but are likely to develop significant disability (GOV.UK 2025; MedlinePlus 2022).
Bart’s Hydrops Fetalis is also dangerous for the pregnant person and may cause complications such as:
- Pre-eclampsia
- Antepartum haemorrhage
- Retained placenta
- Death.
(GOV.UK 2025)
Sickle Cell Disease
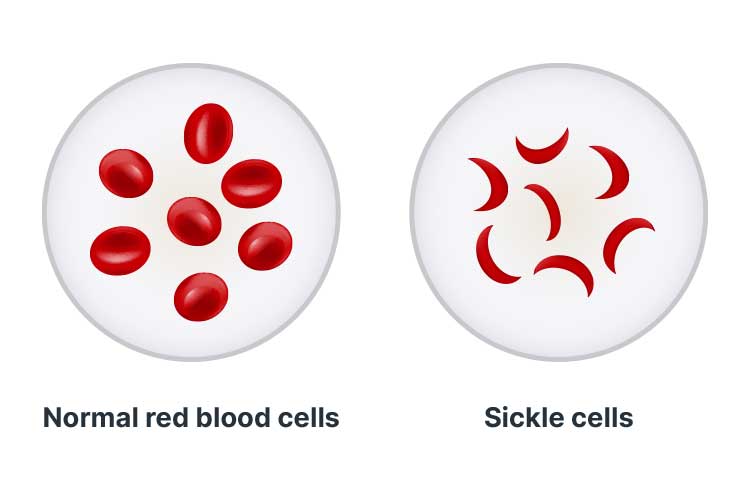
Sickle cell disease (SCD) is an umbrella term describing a group of disorders that cause the production of haemoglobin with an abnormal structure and quality (GOV.UK 2025; CDC 2025).
SCD causes an abnormal process known as sickling, wherein the red blood cells lose oxygen and change shape to resemble a sickle (a farm tool shaped like the letter C) (GOV.UK 2025).
Red blood cells are typically round and can easily manoeuvre through blood vessels. However, cells affected by SCD become hard and sticky, obstructing blood vessels and blood flow (Mayo Clinic 2025).
Normal red blood cells die after 90 to 120 days. However, sickle cells die prematurely (within 10 to 20 days), meaning that there is a constant lack of red blood cells in the body. This causes chronic anaemia (NHLBI 2024).
Symptoms of Sickle Cell Disease
SCD is characterised by vaso-occlusive crises - sudden, potentially life-threatening episodes of pain that usually affect the abdomen, chest, bones or joints. Crises occur when sickle cells obstruct a blood vessel. They may be induced by events such as sudden body temperature changes, dehydration, shortage of oxygen or an infection (Mangla et al. 2023; Healthdirect 2024; GOV.UK 2025).
Other symptoms of SCD include:
- Chronic pain
- Fatigue
- Vision issues
- Slow weight gain/faltering growth/failure to thrive
- Increased risk of infection
- Jaundice.
(Healthdirect 2024)
Complications of Sickle Cell Disease
Potential complications include:
- Acute chest syndrome
- Organ damage (e.g. spleen, liver, kidneys, heart, lungs)
- Stroke (if sickle cells obstruct blood flow to the brain)
- Aplastic crises
- Acute intrahepatic cholestasis
- Sequestration crises
- Infections such as pneumonia
- Slow weight gain/faltering growth/failure to thrive
- Priapisms (prolonged, painful erections)
- Acute or chronic vision issues
- Iron overload
- Necrosis of joints
- Leg ulcers
- Pulmonary artery hypertension
- Chronic kidney disease and other renal complications
- Gallstones
- Deep vein thrombosis
- Pregnancy complications.
(Healthdirect 2024; Mangla et al. 2023; Mayo Clinic 2025)
Treatment of Sickle Cell Disease
SCD can only be cured through a bone marrow or stem cell transplant (GOV.UK 2025).
However, the condition can be managed through:
- Specialist medical care
- Prophylactic antibiotics and immunisation to prevent infection
- Hydroxycarbamide (if deemed medically appropriate)
- Regular blood transfusions
- Analgesic medicines for pain relief during crises
- Annual transcranial doppler scans to check for the risk of stroke
- Maintaining a healthy, balanced diet
- Adequate fluid intake
- Keeping warm.
(GOV.UK 2025)
Test Your Knowledge
Question 1 of 3
Which one of the following conditions is caused by inheriting two abnormal copies of the HBA1 gene and two abnormal copies of the HBA2 gene?
Topics
References
- Better Health Channel 2019, Thalassaemia, Victoria State Government, viewed 26 June 2025, https://www.betterhealth.vic.gov.au/health/ConditionsAndTreatments/thalassaemia
- Centers for Disease Control and Prevention 2025, About Sickle Cell Disease, U.S. Department of Health and Human Services, viewed 26 June 2025, https://www.cdc.gov/sickle-cell/about/
- Australian Living Evidence Collaboration 2025, Australian Pregnancy Care Guidelines, Australian Government, viewed 25 June 2025, https://app.magicapp.org/?language=sl#/guideline/jm83RE
- GOV.UK 2025, Understanding Haemoglobinopathies, Government of the United Kingdom, viewed 25 June 2025, https://www.gov.uk/government/publications/handbook-for-sickle-cell-and-thalassaemia-screening/understanding-haemoglobinopathies
- Healthdirect 2024, Sickle Cell Anaemia, Australian Government, viewed 26 June 2025, https://www.healthdirect.gov.au/sickle-cell-anaemia
- Mangla, A, Agarwal, N & Maruvada, S 2023, ‘Sickle Cell Anemia’, StatPearls, viewed 26 June 2025, https://www.ncbi.nlm.nih.gov/books/NBK482164/
- Mayo Clinic 2024, Autosomal Recessive Inheritance Pattern, Mayo Clinic, 26 June 2025, https://www.mayoclinic.org/autosomal-recessive-inheritance-pattern/img-20007457
- Mayo Clinic 2025, Sickle Cell Anemia, Mayo Clinic, viewed 26 June 2025, https://www.mayoclinic.org/diseases-conditions/sickle-cell-anemia/symptoms-causes/syc-20355876
- MedlinePlus 2022, Alpha Thalassemia, U.S. Department of Health and Human Services, viewed 26 June 2025, https://medlineplus.gov/genetics/condition/alpha-thalassemia/
- National Heart, Lung, and Blood Institute 2024, Sickle Cell Disease: Causes and Risk Factors, National Institutes of Health, viewed 26 June 2025, https://www.nhlbi.nih.gov/health/sickle-cell-disease/causes
- Royal Australian College of General Practitioners 2023, ‘Haemoglobinopathies’, Genomics in General Practice, 3rd edn, RACGP, viewed 25 June 2025, https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/genomics-in-general-practice/disease-specific-topics/haemoglobinopathies
 New
New 
