Workers employed by National Disability Insurance Scheme (NDIS) providers, who deliver supports to NDIS participants requiring subcutaneous injections, must be able to provide appropriate support.
This article has been updated in response to the release of Version 3 of the NDIS Practice Standards: High Intensity Support Skills Descriptors (HISSD), which came into effect on 1 February 2023.
What are Subcutaneous Injections?
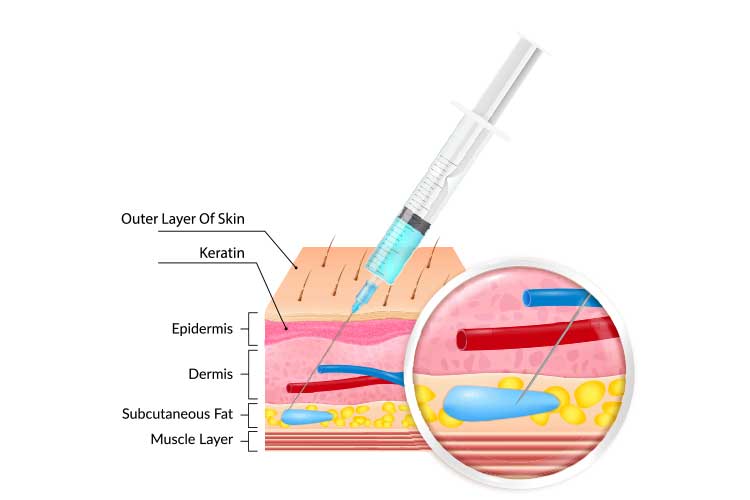
Subcutaneous injections are used to administer medication into the fatty tissue layer between the skin and the muscle (subcutaneous tissue). The subcutaneous tissue has a smaller blood supply, meaning the medication is absorbed more slowly than if inserted into a vein (Case-Lo 2018; Shepherd 2018).
This type of injection may be beneficial for medications such as insulin and heparin that require continuous absorption (Shepherd 2018).
Additionally, subcutaneous injections are a less expensive, easier and sometimes more effective way of administering certain medications (Case-Lo 2018).
Subcutaneous Injections in the NDIS Practice Standards
Subcutaneous Injections are set out in the NDIS Practice Standards under the High Intensity Daily Personal Activities Module.
This Practice Standard aims to ensure that NDIS participants who require subcutaneous injections receive supports that are appropriate, relevant and proportionate to their individual needs (NDIS 2021).
Under these standards, NDIS providers must meet the following quality indicators:
- Participants are enabled to engage in the assessment and development of a subcutaneous injection plan that includes dosage measurement and calculation. This plan identifies possible risks, incidents and emergencies, and what actions need to be taken to manage these situations, including the escalation of care, if necessary. The participant’s health status is reviewed regularly (with the participant’s consent).
- The participant has been provided with a written prescription or phone order prescribing the medication that will be administered subcutaneously. This prescription must be documented.
- Workers providing subcutaneous injection support are informed by appropriate policies, procedures and training plans.
- Workers providing subcutaneous injection support have received all necessary training from a qualified health practitioner or another appropriately qualified individual, and have a basic understanding of the participant’s underlying health condition that requires subcutaneous injections.
(NDIS 2021)
In order to recieve subcutaneous injection support under the NDIS, a participant must have a subcutaneous injection support plan in place. This support plan should be developed in consultation with a health practitioner (NDIS 2022).
The participant’s support plan may outline support needs such as:
- Medication requirements and dose calculations
- Injection procedures
- Issues to look out for
- Required actions to take in response to risks, incidents and emergencies.
(NDIS 2022)
Subcutaneous Injection Skills Descriptors and Knowledge
The NDIS high intensity support skills descriptors are additional guidance specifically for NDIS workers who are supporting participants with high intensity daily personal activities (HIDPA). Many of these HIDPAs are high-risk and/or intimate care areas that require a high level of care, competency and communication. The high intensity support skills descriptors set out the skills and knowledge required for NDIS workers to effectively and safely support participants with HIDPAs (NDIS 2022).
The high intensity support skills descriptors have been updated as of 1 February 2023 in order to:
- Ensure they reflect contemporary practice and expert advice
- Align their format and language more closely to the NDIS Workforce Capability Framework.
(NDIS 2023)
Skills Required for Subcutaneous Injections
Workers providing subcutaneous injection support under the NDIS should be able to:
- Understand and follow the participant’s support plan
- Ask the participant about their expectations, capacity and preferences for being involved in the delivery of their care
- Ask the participant about their communication preferences, and communicate in their preferred way
- Follow hygiene and infection control principles, including hand hygiene, disinfecting the environment and wearing gloves
- Ensure required equipment and consumables (e.g. injecting equipment medications) are available and prepared for use
- Provide support in the least intrusive and restrictive way practicable, in alignment with the participant’s daily routine and preferences
- Support the participant to position themselves so that the injection site is accessible
- Monitor the participant before, during and after the injection and immediately escalate care if they observe an adverse reaction or infection
- Safely use and dispose of sharps and consumables
- Document required information and observations in the participant’s support plan
- Work collaboratively with other members of the care team
- Correctly document medications that have been administered
- Discuss any changes needed to subcutaneous injection support with the participant
- Support the participant to provide feedback or request changes to their support plan.
(NDIS 2022)
Workers supporting a participant to manage diabetes should be able to:
- Understand the participant’s specific support requirements for their diabetes, e.g. medications, method of delivery, procedures and timing
- Ensure the participant has access to glucose monitoring equipment in line with their support plan
- Assist the participant to routinely monitor their blood glucose levels in line with their support plan
- Recognise and respond to hypoglycaemia or hyperglycaemia
- Assist the participant to administer insulin in line with their support plan
- Identify and immediately take action in response to issues such as illness, infection, adverse medication reaction or incorrect dosage administration
- Calculate, draw up and double check medication dosages prior to injection by following the procedures established by an appropriately qualified healthcare practitioner
- Assist the participant with diabetes management to the extent they choose, in line with their support plan.
(NDIS 2022)
Workers supporting a participant with a pre-filled injection should be able to:
- Set up pre-filled pens, pumps and other required equipment in order to administer medication.
(NDIS 2022)
Knowledge Required for Subcutaneous Injection Support
NDIS Code of Conduct and Practice Standards
Read: The NDIS Practice Standards Explained
The NDIS Practice Standards establish the benchmark of performance, quality and safety that NDIS providers should meet in their delivery of supports and services (NDIS 2021).
The Standards work alongside the NDIS Code of Conduct to inform NDIS participants about the quality they should be expecting from the supports and services they receive (NDIS 2021).
The NDIS Code of Conduct sets out expectations for the behaviour of both NDIS workers and participants in order to promote safe and ethical service delivery. For more information of the NDIS Code of Conduct, see https://www.ndiscommission.gov.au/about/ndis-code-conduct.
Communication Supports
Participants may have a variety of communication needs and preferences, such as the use of communication aids or devices, or access to resources in another language (NDIS 2022). Remember to individualise communication to ensure each participant’s needs and preferences are met.
For more information on communication supports, see the following Ausmed resources:
- Communicating Effectively With People With Disability
- Communication Skills
- Communication for Quality Care
Infection Prevention and Control
It’s essential to be aware of and follow infection control principles, including PPE, handwashing and disinfecting (NDIS 2022).
For more information on infection control, see Ausmed’s Training Module on Infection Prevention and Control.
Scope of Practice
Always work within your scope of practice and your role’s responsibilities. Refer to your manager and/or local policies and procedures for more clarity on your specific role and responsibilities if you are unsure.
Also see Ausmed’s Training Module on Scope of Practice: Care Workers.
Diabetes
Some participants require subcutaneous injection support as part of managing their diabetes. Therefore, you will need to have a basic understanding of diabetes and how it affects the participant.
For more information on diabetes, see the resources in Ausmed’s Diabetes Learning Hub.
Equipment Required for Subcutaneous Injections
This includes:
- An injectable medication order
- Injectable device
- Syringes
- Needles (16 mm long and 25 to 27 gauge)
- Swabs
- Sharps container.
(NDIS 2022; AIH 2021)
What Kinds of Medications Can Be Administered Subcutaneously?
- Medications that can be administered in small doses (less than one to two mL)
- Medications that need to be administered quickly
- Medications that need to be administered daily or at home
- Insulin
- Anticoagulant medication
- Some fertility medications
- Autoimmune medications
- Certain hormones
- Analgesics (e.g. morphine)
- Antiemetics
- Some vaccines and allergen immunotherapies.
(Case-Lo 2018; Villines 2018)
Note that medications may have different storage requirements. Ensure you refer to the manufacturer’s instructions as well as your organisation’s policies and prcedures.
Choosing an Injection Site
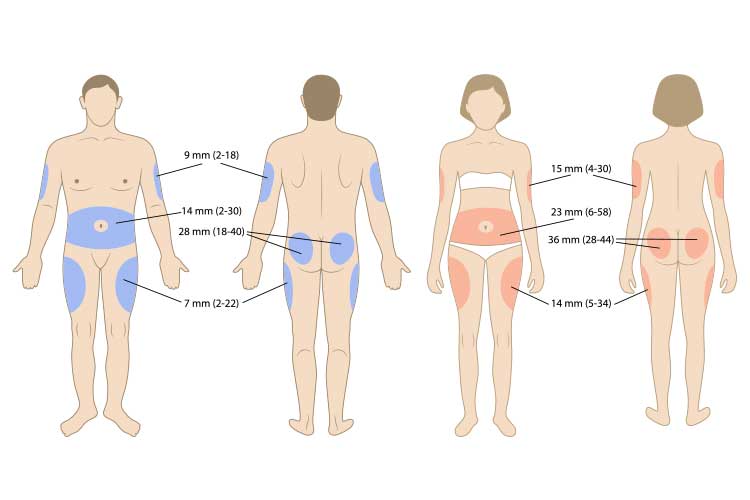
The needle must be injected into a site with a layer of subcutaneous tissue. Recommended areas include:
- Umbilical region of the abdomen, about two inches from the navel (avoid the navel)
- Back or side of the upper arm
- Top of the thigh
- Top of the buttocks.
(Villines 2018; Shepherd 2018; Case-Lo 2018)
Avoid areas of skin that have:
- Infection
- Wounds
- Oedema
- Skin lesions
- Scars
- Birthmarks
- Bony prominences
- Large underlying muscles
- Blood vessels or nerves
- Burns
- Bruises
- Broken skin.
(Shepherd 2018; Queensland Health 2020)
It is important to note that each patient has varying levels of subcutaneous fat, so each individual should be assessed before proceeding with the injection. Lifting the skin fold can help separate the subcutaneous away from the muscle underneath (which should be avoided) (Shepherd 2018).
If the patient requires frequent injections, you should rotate injection sites to allow each area to heal (Villines 2018).
The longevity of each site can range from 1 to 14 days, depending on the type of medication being administered, the type of cannula being used and other factors (Queensland Health 2020).
Method of Injection
- Perform hand hygiene. While the World Health Organisation does not recommend wearing gloves for administering injections, use your own judgement. Wear gloves if bleeding is expected.
- Prepare an appropriate syringe with the required dosage. Disperse any air bubbles.
- Place the participant into a reclined position.
- Choose the injection site.
- Perform hand hygiene.
- If required, cleanse the chosen injection site and wait for it to dry.
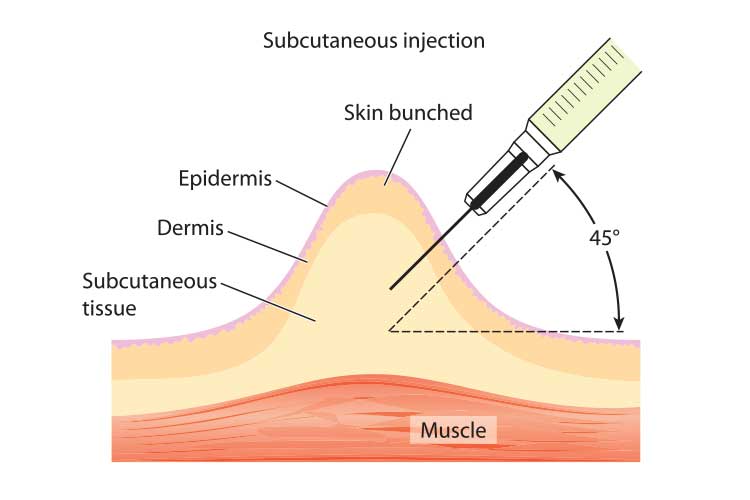
- Hold the syringe with your dominant hand. With your non-dominant hand, lift a 5 cm fold of skin to separate the subcutaneous layer from the muscle tissue underneath.
- Using a quick, dart-like technique, insert the syringe at a 45 to 90-degree angle - follow product information for specific guidelines for each medication.
- Hold the barrel of the syringe firmly and inject the contents for 10 to 30 seconds. The plunger should be pressed all the way down.
- Wait 10 seconds, then remove the needle and immediately dispose of it into a sharps container.
- Do not rub the injection site.
- Apply a dressing if the site bleeds.
- Record the injection using the required documentation.
- Monitor for any adverse reactions or complications.
(QAS 2020; Shepherd 2018; Johns Hopkins Arthritis Center 2012; WHO 2010)
Minimising Anxiety and Discomfort
There are a variety of strategies that can be employed to minimise participant anxiety and discomfort when administering subcutaneous injections. These should be individualised. Examples include:
- Using words like ‘pinch’ or ‘poke’ instead of words like ‘shot’ to describe the procedure
- Performing the procedure in a private, calm and quiet space
- Having the participant’s family member or friend present to comfort or distract them during the procedure
- Offering distractions such as videos during the procedure
- Teaching and encouraging the participant to use relaxation and breath control techniques.
(CDC 2022)
Complications
You should be able to recognise complications of subcutaneous injections and appropriately escalate care. Complications can include:
- Pain
- Bleeding
- Redness
- Swelling
- Warmth
- Tenderness or hardness
- Haematoma
- Leakage
- Infection
- Medication withdrawal
- Medication side effects
- Incorrect medication dosage.
(Case-Lo 2018; Queensland Health 2020; NDIS 2022)
Conclusion
NDIS providers should ensure that staff supporting participants with enteral feeding have the skills and knowledge outlined by the Skills Descriptors, and are reviewed annually for competency (NDIS 2022).
Test Your Knowledge
Question 1 of 3
True or false: The subcutaneous tissue absorbs medication more quickly than the veins.
Topics
References
- Australian Immunisation Handbook 2021, Table. Recommended Needle Size, Length and Angle for Administering Vaccines, Australian Government, viewed 20 June 2023, https://immunisationhandbook.health.gov.au/resources/handbook-tables/table-recommended-needle-size-length-and-angle-for-administering-vaccines
- Case-Lo, C 2018, ‘What Is a Subcutaneous Injection?’, Healthline, 17 September, viewed 20 June 2023, https://www.healthline.com/health/subcutaneous-injection
- Centers for Disease Control and Prevention 2022, Healthcare Providers: Understanding Needle Fears and Phobia, U.S. Department of Health & Human Services, viewed 21 June 2023, https://www.cdc.gov/ncbddd/humandevelopment/covid-19/needle-phobia/healthcare-providers.html
- Johns Hopkins Arthritis Center 2012, How to Give a Subcutaneous Injection, Johns Hopkins Arthritis Center, viewed 21 June 2023, https://www.hopkinsarthritis.org/patient-corner/how-to-give-a-subcutaneous-injection/
- NDIS Quality and Safeguards Commission 2022, NDIS Practice Standards: High Intensity Support Skills Descriptors, NDIS Quality and Safeguards Commission, viewed 21 June 2023, https://www.ndiscommission.gov.au/providers/registered-ndis-providers/provider-obligations-and-requirements/ndis-practice-standards-1
- NDIS Quality and Safeguards Commission 2021, NDIS Practice Standards: NDIS Practice Standards and Quality Indicators, NDIS Quality and Safeguards Commission, viewed 21 June 2023, https://www.ndiscommission.gov.au/providers/registered-ndis-providers/provider-obligations-and-requirements/ndis-practice-standards-1
- NDIS Quality and Safeguards Commission 2023, New NDIS Practice Standards and Quality Indicators, NDIS Quality and Safeguards Commission, viewed 21 June 2023, https://www.ndiscommission.gov.au/providers/registered-ndis-providers/provider-obligations-and-requirements/ndis-practice-standards-1
- Queensland Ambulance Service 2020, Clinical Practice Procedures: Drug Administration/Subcutaneous, Queensland Government, viewed 21 June 2023, https://www.ambulance.qld.gov.au/docs/clinical/cpp/CPP_Subcutaneous.pdf
- Queensland Health 2020, Management of Subcutaneous Infusions in Palliative Care, Queensland Government, viewed 21 June 2023, https://www.health.qld.gov.au/cpcre/subcutaneous/section3
- Shepherd, E 2018, ‘Injection Technique 2: Administering Drugs via the Subcutaneous Route’, Nursing Times, vol. 114, no. 9, viewed 20 June 2023, https://www.nursingtimes.net/clinical-archive/assessment-skills/injection-technique-2-administering-drugs-via-the-subcutaneous-route-28-08-2018/
- Villines, Z 2018, ‘Is a Subcutaneous Injection Painful?’, Medical News Today, 8 August, viewed 20 June 2023, https://www.medicalnewstoday.com/articles/322710
- World Health Organisation 2010, WHO Best Practices for Injections and Related Procedures Toolkit, WHO, viewed 21 June 2023, https://www.who.int/publications/i/item/9789241599252
 New
New