This article will look at how to monitor and assess a patient receiving a blood transfusion.
What is a Blood Transfusion?
Blood transfusion is the transfer of blood components from one person to another. It is a potentially life-saving procedure that helps replace blood lost due to surgery, illness, bleeding or severe injury (Mayo Clinic 2022).
Blood Components
There are several blood components. These include:
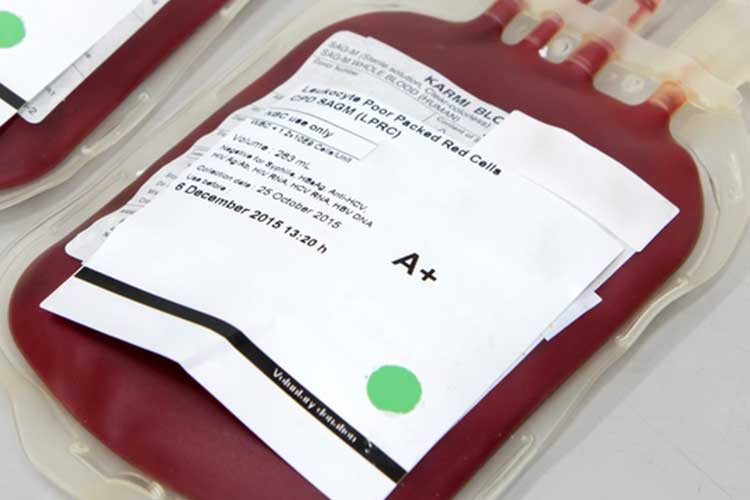
Red Cells
These are required to increase the oxygen-carrying capacity of the blood (Lifeblood 2021a).
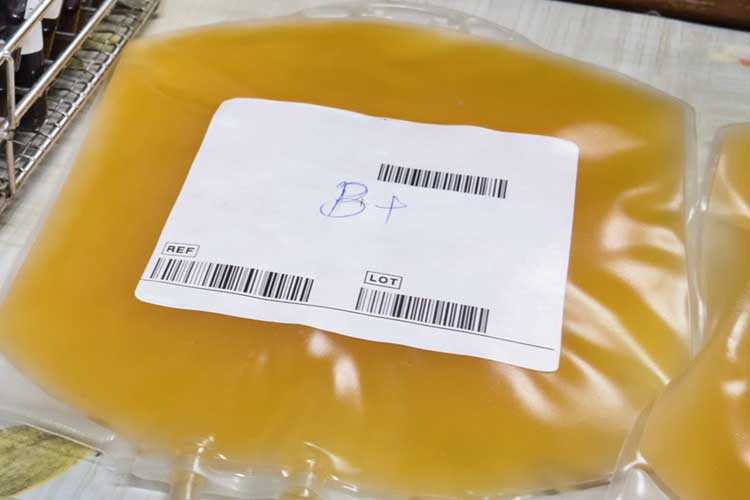
Plasma
The liquid part of blood. It is transfused as fresh frozen plasma (FFP) (Lifeblood 2021b).

Platelets
These are required for the prevention and treatment of haemorrhage in patients with platelet function defects (Lifeblood 2021c).
The Importance of Patient Identification
All patients receiving a blood transfusion must wear a patient identification band. In Australia, at least three of the following approved identifiers must be used:
- Name (family and given names)
- Address
- Date of birth
- Gender
- Medical record number (MRN)
- Individual healthcare identifier.
(ACSQHC 2019)
This information must be legible and accurate.
In an emergency situation, patient identifiers may be unknown. In this situation, the patient should be labelled as ‘unknown male’ or ‘unknown female’ using an emergency MRN or National Health Index (NHI) number. Remember to follow local policies and procedures (ANZSBT 2024).
Patient identification should be checked and confirmed as correct at each stage of the transfusion process. Whenever possible, the patient should be asked to state their full name and date of birth. These must exactly match the information on the patient’s wristband and any other associated paperwork required at that stage of the blood transfusion process.
For patients who are unable to respond entirely or are unconscious or confused, verification of the patient’s identification should be obtained from a parent or carer if present.
If patient identification discrepancies occur at any stage of the transfusion process, the information must be verified, and discrepancies investigated and corrected before proceeding to the next stage (Aneskey 2016).
Blood Transfusion Documentation
Complete documentation is required at every stage of the blood transfusion process and should include the following steps:
1. Pre-transfusion:
- Clinical indication for transfusion
- Date of decision
- Informed consent from the patient based on a discussion of the potential benefits and risks of the procedure
- Blood component to be transferred and volume.
2. Administration:
- The date and time components were collected
- Date and time transfusion started
- The donation or batch number of the components transfused
- Volume administered
- Identification of staff who administered the transfusion
- Observations before and during transfusion.
3. Post-transfusion:
- The date and time the transfusion was completed
- Evidence of the fate of the components
- An indication of whether transfusion achieved the desired effect
- Observations before, during and after transfusion
- Documentation of any reactions that occurred.
(O’Reilly 2020; Lifeblood 2021d)
Traceability
All blood components should be traceable from the donor to their final destination. Follow your organisation’s policies on how to achieve this.
Equipment
Equipment used during blood transfusion should include the following:
- Standard peripheral intravenous cannula, central line or PICC line.
- Blood administration set:
- Blood components must be administered using a blood administration set.
- To prevent bacterial growth, the blood administration line should be changed at least every 12 hours, or after completion of the prescribed blood transfusion.
- Platelets should not be transfused through an administration set that has previously been used for red cells or other components because this may cause platelet aggregation and retention in the line.
- Blood warmers:
- Rapid infusion of red cells soon after their removal from blood refrigeration can lead to hypothermia in surgical or trauma patients.
- Blood should only be warmed using specially designed and regularly maintained blood-warming equipment. Blood must never be warmed in a microwave, with hot water or on a radiator.
(ANZSBT 2024)
Monitoring a Patient Receiving a Blood Transfusion
1. Pre-transfusion:
- Transfusion observations (heart rate, temperature, blood pressure and respiration rate) must be clearly distinguished from other routine observations and should be recorded in the patient’s clinical notes. This is to provide baseline observations to ensure prompt recognition and timely intervention should an adverse effect occur.
2. Administration:
- The patient’s vital signs should be monitored and recorded 15 minutes after commencing the administration of each blood component pack. For the remainder of the transfusion, follow your organisation’s policy on how often vital signs should be measured.
- Patients should be involved in their care; they should be well-informed of the potential risks of undergoing the transfusion because they may be the first to become aware of any adverse reactions. They should also be advised to report any adverse effects (the call bell should be within reach) and should be in an environment where they can be visually observed.
3. Post-transfusion:
- Record the post-transfusion vital signs after each blood component has been transfused. Any routine observation should be continued, especially if the patient is critically ill.
(Lotterman & Sharma 2023; ANZSBT 2024)
Adverse Reactions to a Blood Transfusion
Read: Blood Transfusion Reactions
Conclusion
Full documentation must be completed at every stage of the blood transfusion in the patient’s clinical records. Patients should also be monitored throughout their blood transfusion to ensure quick identification of any adverse effects.
Test Your Knowledge
Question 1 of 3
When should a patient’s vital signs be monitored after starting the administration of a blood component pack?
Topics
Further your knowledge
 Free
Free Free
FreeReferences
- Anesthesia Key 2016, Monitoring a Patient Receiving a Blood Transfusion, Aneskey, viewed 23 October 2024, https://aneskey.com/monitoring-a-patient-receiving-a-blood-transfusion/
- Australian Commission on Safety and Quality in Health Care 2019, Action 6.05: Correct Identification and Procedure Matching, Australian Government, viewed 23 October 2024, https://www.safetyandquality.gov.au/standards/nsqhs-standards/communicating-safety-standard/correct-identification-and-procedure-matching/action-605
- Australian and New Zealand Society of Blood Transfusion 2024, Guidelines for the Administration of Blood Products, 3rd edn, ANZSBT, viewed 23 October 2024, https://anzsbt.org.au/wp-content/uploads/2024/02/Guidelines-for-the-Administration-of-Blood-Products-revised-Feb-2024.pdf
- Lifeblood 2021a, Red Cell Transfusions, Australian Red Cross Lifeblood, viewed 22 October 2024, https://www.lifeblood.com.au/patients/types-of-transfusions/red-cells
- Lifeblood 2021b, Plasma Transfusions, Australian Red Cross Lifeblood, viewed 22 October 2024, https://www.lifeblood.com.au/patients/types-of-transfusions/plasma
- Lifeblood 2021c, Platelet Transfusions, Australian Red Cross Lifeblood, viewed 22 October 2024, https://www.lifeblood.com.au/patients/types-of-transfusions/platelets
- Lifeblood 2021d, Documentation and Traceability, Australian Red Cross Lifeblood, viewed 23 October 2024, https://www.lifeblood.com.au/health-professionals/clinical-practice/transfusion-process/documentation-traceability
- Lotterman, S & Sharma, S 2023, ‘Blood Transfusion’, StatPearls, viewed 23 October 2024, https://www.ncbi.nlm.nih.gov/books/NBK499824/
- Mayo Clinic 2022, Blood Transfusion, Mayo Clinic, viewed 22 October 2024, https://www.mayoclinic.org/tests-procedures/blood-transfusion/about/pac-20385168
- O’Reilly, C 2020, ‘Chapter 9 Blood Administration’, in Khandelwal, A & Abe, T (eds), Clinical Guide to Transfusion, viewed 23 October 2024, https://professionaleducation.blood.ca/en/transfusion/clinical-guide/blood-administration
 New
New 
