What are Medical Devices?
Medical devices are essential for diagnosing, preventing, treating, and monitoring medical conditions. They include a wide range of products, from surgical tools and syringes to pacemakers and implants, just to name a few. Understanding these devices is crucial for healthcare professionals, as they directly impact patient care (Department of Health and Aged Care, Therapeutic Goods Administration 2024).
The Importance of Medical Devices in Healthcare
No medication or medical device is entirely free of risks and side effects. We must take various measures to minimise these potential risks. Medical devices are vital to modern medicine, providing support in almost every aspect of patient care. From diagnosis to treatment and ongoing patient treatment, medical devices enhance the capability of healthcare professionals to treat and manage a range of conditions. Their importance extends beyond direct patient care, contributing significantly to research and developing new medical techniques and treatments. Proper use of these devices directly correlates to improved patient outcomes and advancements in healthcare (Department of Health and Aged Care, Therapeutic Goods Administration 2024).
(Australian Health Journal 2024)
What is the "Medical Devices" Training Requirement?
Training in the use of medical devices is a critical component of healthcare education. It ensures that healthcare professionals are proficient in using these devices, necessary for patient safety and effective treatment. The training encompasses understanding medical device functionality, handling, maintenance, and sterilisation. It also includes training on how to respond to emergencies involving device malfunction. Adherence to standards set by the NSQHS and Aged Care Quality and Safety Commission is essential in this training, ensuring that healthcare providers are up-to-date with best practices and regulations.
Healthcare professionals must be skilled and demonstrate competence in operating various medical equipment. For instance, in critical care settings, ventilators are vital for patients who need mechanical ventilation to facilitate breathing; improper use can lead to severe complications or even death. Similarly, healthcare professionals must use blood pressure machines correctly obtain and interpret data to diagnose and manage various health conditions. It is vital that health professionals operate medical devices within their scope of practice to provide safe care.
Relevant Standards
Action 3.12: Invasive medical devices
The health organisation implements processes for the use and management of invasive medical devices consistent with the current version of the Australian Guidelines for the Prevention and Control of Infection in Healthcare
Action 3.17: Reprocessing of reusable equipment and devices
When reusable devices and equipment are used, the health organisation has:
- Reprocessing systems consistent with national and international standards in conjunction with manufacturer guidelines
- A process to trace critical and semi-critical devices, instruments and equipment that is capable of identifying the patient, procedure, and the equipment, instruments, and devices used for the procedure
- Processes to manage and plan reprocessing requirements and additional control for novel and emerging infections
National Safety and Quality Health Service (NSQHS) Standards
Action 5.2.2: Minimising and managing infection in clinical care by:
b. using, managing and reviewing invasive devices, including urinary catheters
Strengthened Quality Standards framework analysis - Aged Care Quality Standards
Related Training Requirements Guides
The following Training Requirement guides can be used to support and facilitate the "medical device use and repurposing" training requirement:
Skills Required for Medical Device Use and Repurposing
Staff feedback and complaints management must demonstrate effective communication, empathy, problem-solving, and a thorough understanding of healthcare policies and procedures. These skills are crucial for appropriately addressing and resolving issues raised by patients and their families.
- Device-specific knowledge: Staff must understand each device's purpose, mechanics, and operational procedures. This includes correctly setting up, operating, and troubleshooting invasive devices like ventilators, dialysis machines, or surgical tools.
- Application techniques: Staff must be skilled in the precise techniques of applying these devices, such as correctly inserting an indwelling catheter.
- Cleaning: It is important to understand cleaning protocols for different types of invasive devices thoroughly. This skill prevents infection and cross-contamination and ensures device longevity.
- Sterilisation procedures: Staff trained in sterilisation should be well-versed in sterilising equipment and thoroughly understand the different methods and their applications to various materials and types of devices.
- Storage and handling: Appropriate techniques to maintain device integrity and prevent contamination.
- Emergency protocols: Ability to demonstrate emergency procedures in case of device failure, including backup plans and alternative treatment options.
- Accurate record keeping: Maintaining detailed records of device usage, patient reactions, and any issues encountered.
- Regulatory compliance: Understanding and adhering to all relevant guidelines and regulations regarding invasive medical devices.
How to Assess Staff Competency in Medical Device Use and Repurposing
Assessment of staff competency can be done through:
- Assessment: Explore various techniques for assessing staff competency, including simulations and peer reviews.
- Competency frameworks: Discuss competency frameworks and how they can be applied to medical device training.
- Continuing professional development:Emphasise the importance of continuous learning and development.
Strategies to Support Healthcare Staff Develop Skills in Feedback and Complaints Management
Supporting staff in developing their skills involves:
- Mentorship programs: Describe how mentorship programs can enhance in skill development.
- Online learning: Discuss the role of e-learning platforms and online resources in continuing education.
- Feedback: Explain the importance of feedback in the learning and how it can be effectively implemented in the workplace.
Sample Training Plan for the Medical Device Use and Repurpose Requirement
A structured training plan is essential for developing quality improvement skills.
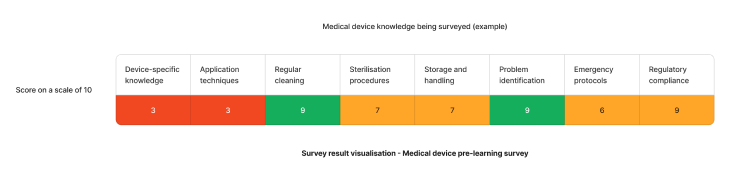
Using the above needs assessment survey as an example - The skills requiring the most attention for medical devices are device-specific knowledge and application techniques. We can target learning to fill these gaps and enhance staff competency.
| Quarter | Topics | Resources |
|---|---|---|
| Q1 | Device-specific knowledge and application techniques |
|
Need an LMS that can support medical device use training?
Contact Ausmed today and see how we can support your training requirement needs!
Staff Competency Assessment for Using and Repurposing Medical Devices Management - Example
Consider the following survey questions to evaluate staff feedback and complaints management skills:
Staff Survey - Medical Device Use and Repurposing Competency
-
How would you rate your comfort using invasive medical devices?
- [Answer here]
-
Describe a situation where you had to troubleshoot a medical device issue.
- [Answer here]
-
What steps do you follow to sterilise reusable medical devices?
- [Answer here]
-
Can you outline the procedure for emergency handling of a malfunctioning medical device?
- [Answer here]
Conclusion
Developing a comprehensive training program for medical devices is crucial for healthcare organisations. This program should not only focus on the operational aspects of using these devices but also emphasise maintenance, troubleshooting, and emergency procedures. Ensuring healthcare staff are well-equipped to handle these devices safely and effectively for delivering high-quality patient care.
References
- Australian Commission on Safety and Quality in Health Care, 2023. 'Preventing and Controlling Infections Standard'
- Australian Commission on Safety and Quality in Health Care, 2023. 'NSQHS Action 3.12'
- Australian Commission on Safety and Quality in Health Care, 2023. 'NSQHS Action 3.17'
- Aged Care Quality and Safety Commission, 2023. 'Stronger Standards, Better Aged Care Program - Action 5.2.2 (b)'
- Australian Health Journal 2024, Ensuring Stringent Quality Standards in the Lifecycle of Medical Devices, online video, 17 April, viewed 26 August 2024, https://www.youtube.com/watch?v=TgwvqMILByI